Introduction: Immune thrombocytopenia (ITP) is a feature of systemic lupus erythematosus (SLE) in 5-20% of cases. ITP may present at the time of SLE presentation or may precede SLE diagnosis by up to nearly a decade. Even in the absence of an SLE diagnosis, ITP patients may have a positive ANA in ~20% of cases.
Some studies suggest that a positive ANA in ITP may herald chronic and more refractory disease, while other studies have not demonstrated a link between ANA+ ITP and progression to SLE. The natural history of ANA+ pediatric ITP patients remains unclear. With the concept that hydroxychloroquine may modulate progression to systemic autoimmunity, European hematologists report using hydroxychloroquine in ANA+ pediatric ITP patients with titers as low as 1:160. However, whether ANA+ ITP patients represent a distinct cohort clinically and should receive different therapies is not well established. The goals of this study were to evaluate the significance and degree of a positive ANA titer at presentation in a cohort of pediatric ITP patients.
Methods: Data from a retrospective cohort of 711 ITP patients treated at Texas Children's Hospital from 2012-2022 were reviewed. Patients were defined as SLE if diagnosed as such by rheumatology. To focus on the prognostic value of an ANA upfront, the cohort was refined to include only ITP patients with ANA testing within the newly diagnosed period (0-3 months). Individuals who received IVIG prior to ANA testing were coded as having a missing ANA result given the possibility of false positive results. Being diagnosed concurrently with SLE and ITP was defined as diagnoses within 1 month of each other. Fisher's exact tests were used to compare nonparametric data with a P <0.05 defined as statistically significant. P values were corrected for multiple testing.
Results: One hundred sixty-one ITP patients were included for analysis. The cohort was an average of 11.9 years-of-age with a slight female predominance (58.4%). Most of the population self-identified as White and non-Hispanic (84.7%, 45.5%). Nearly 80% had primary ITP and 66.7% were ultimately diagnosed with chronic ITP (cITP). The mean follow-up time for the cohort was 2.2 years (with a range from 0 to 12 years).
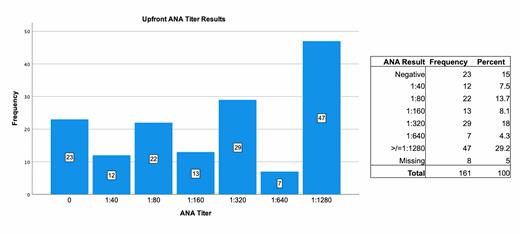
Forty-seven of 161 (29.2%) individuals had a high titer ANA ³1:1280 at ITP presentation. (Figure 1) There was a statistically significant difference in ANA titer and presence of secondary ITP (P<0.001). Sixty-eight percent (n=23) of secondary ITP patients had a high titer (≥1:1280) ANA versus 20% (n=24) of the primary ITP patients. There was no difference between ANA titer and development of cITP vs. non-cITP.
Of this cohort, 20 patients had an ANA tested at ITP presentation and were diagnosed with SLE by rheumatology. Most of these patients had an ANA titer ≥1:1280 (84.2%). The remaining 3 patients had titers of 1:320. Of this cohort, 9 patients had SLE and ITP diagnosed concurrently and 2 had SLE preceding ITP diagnosis by an average of 3.8 years. All 11 patients had an ANA ≥1:1280. Of the 9 who were not diagnosed with SLE at ITP presentation, 6 (66.6%) had an ANA ≥1:1280 and 3 had a titer of 1:320. The lead time to being diagnosed with SLE after presenting with ITP was 1.76 years.
Discussion and Conclusions: In this cohort, high titer ANAs in newly diagnosed ITP patients were linked to development of secondary ITP and SLE. Lower titer ANAs were less common, even in those who went on to develop SLE. These findings suggest that specifically high titer ANA results may herald SLE development in pediatric ITP patients.
To allow the onset of SLE to be identified earlier, individuals with a high titer ANA at ITP presentation may warrant closer follow-up to monitor for progression to systemic autoimmune disease. This subset of ITP patients could benefit from earlier institution of SLE-directed therapy, such as hydroxychloroquine.
Further analysis is underway, including expansion of this analysis to multiple sites within the Pediatric ITP Consortium of North America, as well as surveying pediatric ITP treaters on their practice regarding ANA testing and management in ITP patients.
Disclosures
Macmath:National Institutes of Health: Current Employment. Grimes:Novartis: Research Funding; Sobi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal